Table of Contents
By Dr. René Floris, NIZO’s Chief Innovation Officer and Patrick Janssen, NIZO’s Fermentation Expert.
So, you have a great idea for a new fermentation-based product or ingredient and a successful process in the lab. All that remains is to scale up to mass production. That’s just a matter of running your new fermentation process in larger and larger volumes, maybe tweaking a few parameters along the way, right? Wrong. The physics and economics of fermentation at volume mean the path from lab to factory isn’t straightforward. But by understanding commercial fermentation processes, you can navigate that path more quickly and successfully.

Fermentation is a hot topic across the food industry. Innovative ideas for products and ingredients are emerging, often based on novel or engineered microorganisms. But innovative ideas and novel microbes often mean innovative and novel fermentation processes. And successfully scaling up such processes remains a big challenge.
The traditional approach is to apply a working lab-scale process in successively larger fermentors. identifying and solving any problems that arise before moving on to the next volume step. However, this can lead to double working, as you may have to solve similar problems in new ways at each step. There is also a risk some serious issues won’t arise until the largest volumes, potentially delaying or even stopping development after you have already invested significant amounts of time and money.
Scaling up by scaling down
According to NIZO fermentation expert Patrick Janssen: “You can avoid these pitfalls by looking at how commercial-scale fermentation processes work and, in particular, the constraints they face. Mimicking these constraints at smaller scales helps you make process choices that will transfer more successfully to larger volumes.”
Testing these choices at smaller volumes is quicker and cheaper than waiting until an issue arises at higher volumes, making upscaling more efficient. Moreover, these small-scale trials produce high-quality data that is ideal input for process modelling, for example through a digital twin, which can significantly increase the chances of first-time-right process development.
Perhaps the most obvious constraint is the sheer volume of industrial-scale fermentors and its impact on process timings. For example, heating and cooling times play almost no role in lab-scale processes.
“But it can take 2 hours for a typical industrial fermentation vessel to cool down and 10 hours to empty it,” Janssen points out. “If your process needs immediate cooling to stop fermentation at a specific point, then it is just not going to work at industrial scales. But if you already think about how cooling the vessel slowly in the lab, you will have a much smoother transition to the factory.”
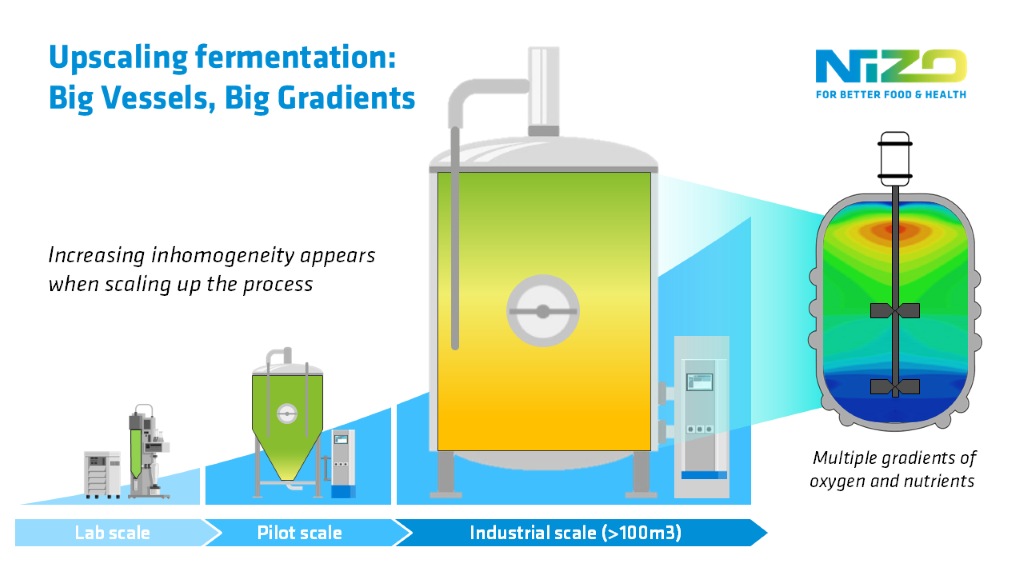
Leaning into gradients
The size of industrial fermentors also means it is practically impossible to control conditions with the same level of precision and homogeneity as in the lab. The temperature within a lab vessel can usually be controlled to within fractions of a degree. But in an industrial fermentor, the temperature can easily vary by a few degrees.
And it isn’t just temperature that varies. In an aerobic process, for instance, you are likely to have higher oxygen concentrations at the bottom and higher nutrient concentrations at the top with a gradient of both oxygen and food levels in between. This can also lead to similar gradients in temperature and parameters such as salt levels and pH.
These factors impact the growth rate and overall yield of your microorganisms, making it difficult to know when to stop the fermentation. This unpredictability would be a real problem for production planning and developing a successful industrial process, which rely on knowing when the microorganisms will move to different steps in the factory.
“You may be able to counteract the effect of these gradients – at least partially – through periodic stirring,” adds Janssen. “But that doesn’t always work. So, you need to test how your microorganism responds to these gradients. If they are going to be a problem, you can check whether stirring will help and if your microbe is physically robust enough to withstand stirring. Doing this early minimizes your risks in the factory.”
Volume processes are built different
Scale isn’t the only difference between industrial and laboratory processes. Another question is how to choose and prepare the growth medium. In the lab, the growth medium is usually batch sterilized before fermentation. This isn’t feasible in the factory, where you are looking for a continuous flow of growth medium. Instead, industrial processes typically use UHT-type steps for sterilization.
Batch sterilization has a higher heat load than UHT, and can cause chemical changes in the growth medium via Maillard reactions. In some circumstances, these can create new compounds that are beneficial to the fermenting microorganism. If those compounds are then not created in the industrial process, you could suddenly be confronted with a much lower growth rate in the factory, delaying development and possibly your market introduction while you search for the cause.
“We have seen numerous examples where Maillard reactions during batch sterilisation have created compounds in the growth medium that stimulated the growth of the fermenting organism,” Janssen explains. “When the processes transferred to the factory and UHT sterilization, those beneficial compounds weren’t there. The organism grew much more slowly and, in some cases, not at all.”
Hitting roadblocks like this at such a late stage can significantly delay the market introduction of new products and cost a great deal of time and money while the cause of the issue is identified and solved.
Industrial thinking in the lab
Developing fermentation processes in the lab is about understanding your microorganism and its needs. But by considering how industrial processes work and why volume facilities make the choices they do, you can start to marry the needs of your microorganism and the needs of a successful industrial process.
Through that insight and forward thinking – perhaps in combinations with AI and predictive modelling tools – you can identify the key steps of your process and where it will clash with the constraints of volume production. Mimicking the impact of those constraints at low volumes will help you upscale your processes faster and more effectively, so you can get to market faster.
This article has also been published on Food Navigator – read more here.
Related cases
Related blogs
© NIZO 2025 | Sitemap - Privacy Statement - Cookie Statement - Terms & Conditions
Website by: Online Marketing Agency