Dairy & Plant Proteins
Proteins are essential for a person's body and needs a balance of all amino acids to function correctly.
A complete protein source can consist of both animal and plant protein. The use of alternative proteins offers a perfect solution to a more sustainable food production system.
Customers are ready to embrace this trend, but will only do so if taste, texture and health remain uncompromised. We can meet their expectations!
Learn more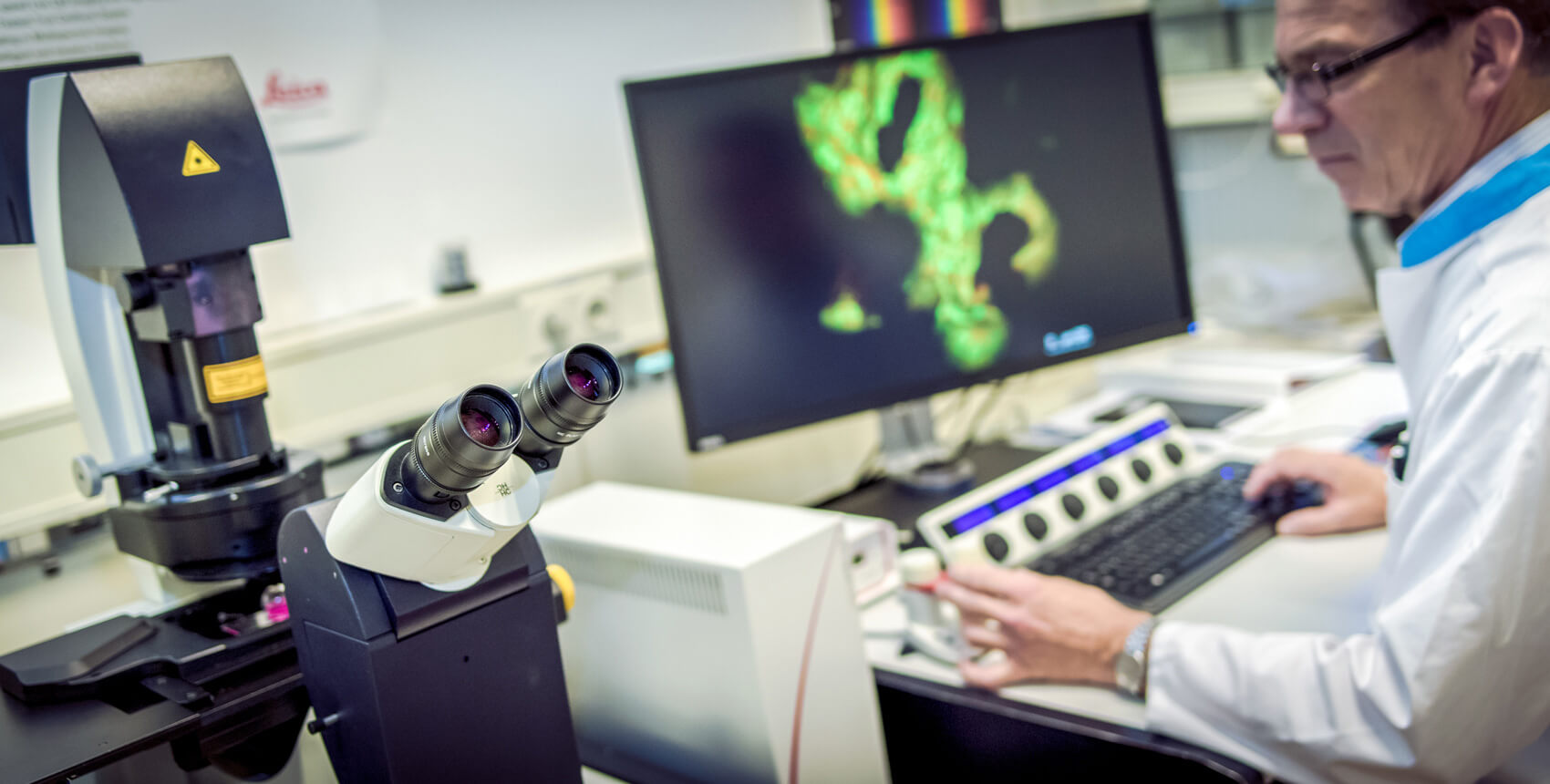

Fermentation & Probiotics
Understanding the diversity and functions of microorganisms is essential for improving your food or other products influencing human health. And use of new strains or other formulations can increase the shelf life and/or safety of the food products or reduce costs of the production process. Optimizing these processing conditions can lead to a significant cost reduction.
Learn morePreclinical & Clinical models
With NIZO’s nutritional research we are able to support qualifying your health claims such as investigating resistance to infection, microbes & health and digestion and gut comfort.
Learn more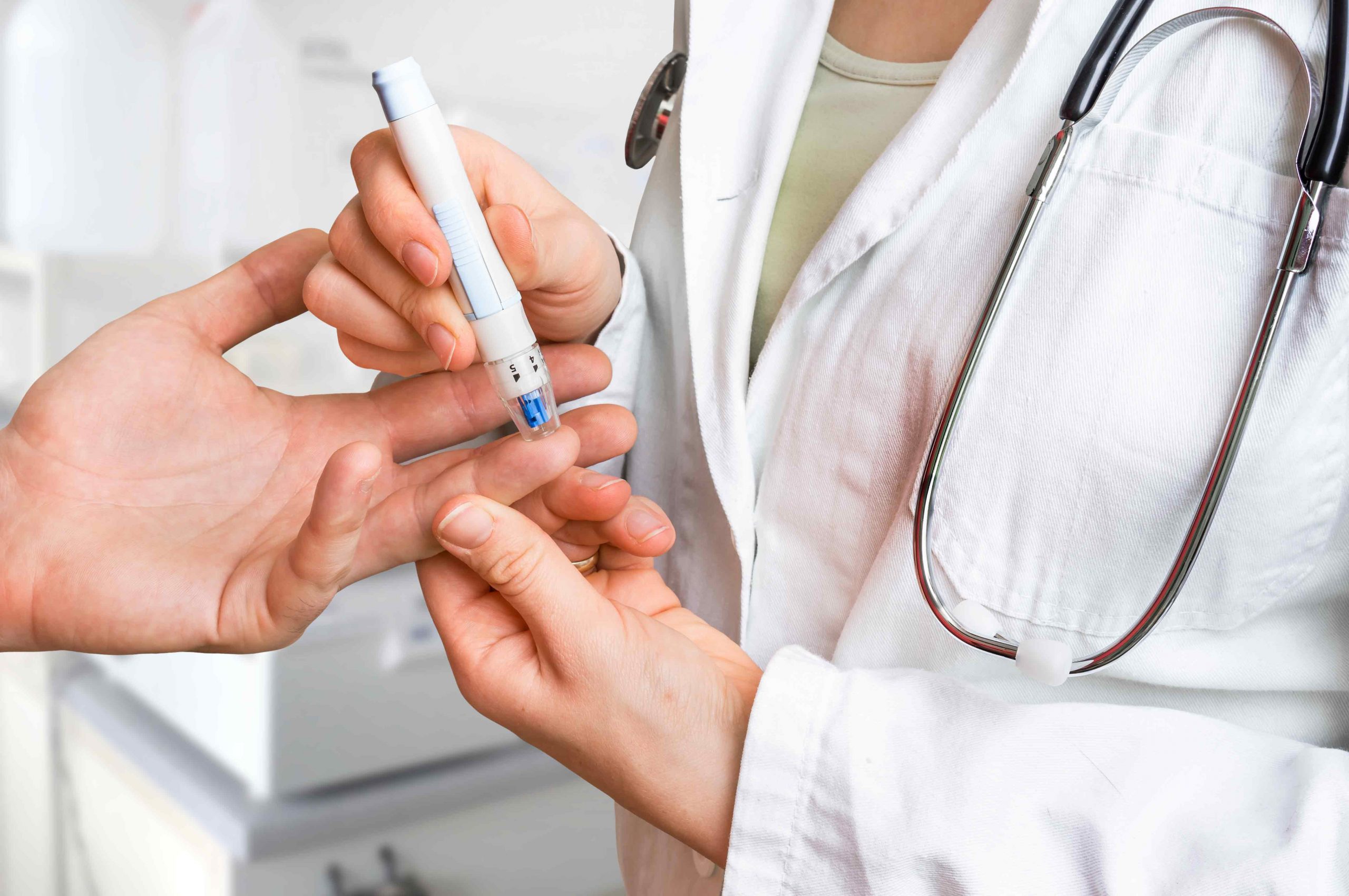

Process Performance and pilot plant manufacturing
Are you optimizing existing processes, designing new production processes or looking for alternative applications for product waste streams? We can assist you with practical solutions, applying our extensive knowledge on proces-product interactions. We offer an extensive pilot facility, covering upstream and downstream processing.
Learn moreFood analytics for research & development
Keep your food product development on the right track, with technical and sensory analytics that help you create foods that meet consumer and quality needs Whether you are developing a food product that should mimic the taste, smell or texture of another, or creating a completely new food to meet consumer demand, you need to be sure it offers the benefits and characteristics you are looking for. Composition. Performance. Quality.
Learn more
Cases
© NIZO 2025 | Sitemap - Privacy Statement - Cookie Statement - Terms & Conditions
Website by: Online Marketing Agency