Table of Contents
In this series of articles, Will Chu, Science Editor for NutraIngredients, discusses some of the key issues and challenges facing the nutraceutical and food ingredient industry today.
Consumer interest in products offering immune health benefits continues to rise, and the Covid-19 pandemic has only increased awareness of immunity issues. But developing an ingredient that offers an immune health benefit in a food or supplement is a long and complex process. Ruud Albers, founder and CSO of NutriLeads, describes his company’s journey to take an innovative, effective and sustainable ingredient from discovery to commercialisation.
WH: How did you get started on the journey to identify, develop and then launch NutriLeads’ immune health ingredient?
RA: Our journey began around 15 years ago. It was already clear at that time that there was a gap in the market to meet consumer demand for products that could enhance immune health and, more specifically, resistance to respiratory tract infections.
The main function of the immune system is to defend our body against infections. In that respect, the immune system resembles a ‘fire brigade’ of the body: most of the time, it waits vigilantly while preparing for the next challenge. Then, when an infection occurs, it must respond quickly and adequately. To do that, it needs more than the ‘right’ number of firefighters, fire trucks, hoses with specific nozzles, etc. It also requires continuous training.
Likewise, the immune system needs essential resources including vitamins and minerals, and it must train to be as effective as possible when an infection occurs. So, we were looking to develop an ingredient that would help the immune system to be at its best when it matters most.
Most immune health compounds available in foods or supplements aim to support a healthy immune system by addressing any deficiencies in vitamins, minerals or energy. This is of course very important, but we wanted to find a more innovative way to promote immune health.
WH: What are the challenges of developing an ingredient that offers immune health benefits?
RA: Many of the challenges are similar for health ingredients in general: the supply chain needs to be sustainable and economically viable, and the ingredients must be safe, easily applicable and (ideally) compatible with a clean label. The specific challenge for immune health comes from the lack of a generally accepted biomarker that can serve as a yardstick. This means that immune markers or in vitro assays in isolation are not very informative for predicting how well the immune system will deal with an infection. You need a more integrative approach.
WH: How did you identify potential compounds with immune health effects?
RA: We started by making an inventory of what was already available. We reviewed leads for presumed immune health ingredients from the scientific literature and popular press, as well as from traditional practices such as Traditional Chinese Medicine and Ayurveda.
Our resulting list had over 470 possible ingredients. By evaluating them for evidence of beneficial effects on the immune system based on previous clinical trials, we reduced the candidates to a top 15, which we took into in vitro testing. This enabled us to identify a traditional extract that had been shown to reduce incidence and severity of naturally occurring respiratory tract infections. The extract also modulated immune function markers in clinical trials, and outperformed other ingredients in in vitro tests.
WH: How did you develop that traditional medicine extract into an ingredient?
RA: In fact, we didn’t! The extract was prepared from ginseng root, which takes 5 years to grow. This meant developing a sustainable supply chain for application as a food ingredient wasn’t feasible.
Instead, we zoomed in on the active ingredient, and discovered that the immune effects are due to a specific soluble fibre. Our breakthrough was when we found that we could also obtain this fibre from carrots, which offer many supply chain possibilities.
We have since carried out tests to ensure the compound’s safety, and to demonstrate stability as well as compatibility with a wide range of product formats. In the meantime, we have also upscaled production from lab-, to pilot plant-, to commercial-level. Today, we produce BeniCaros® by upcycling carrot pomace, a side stream of carrot juice production.
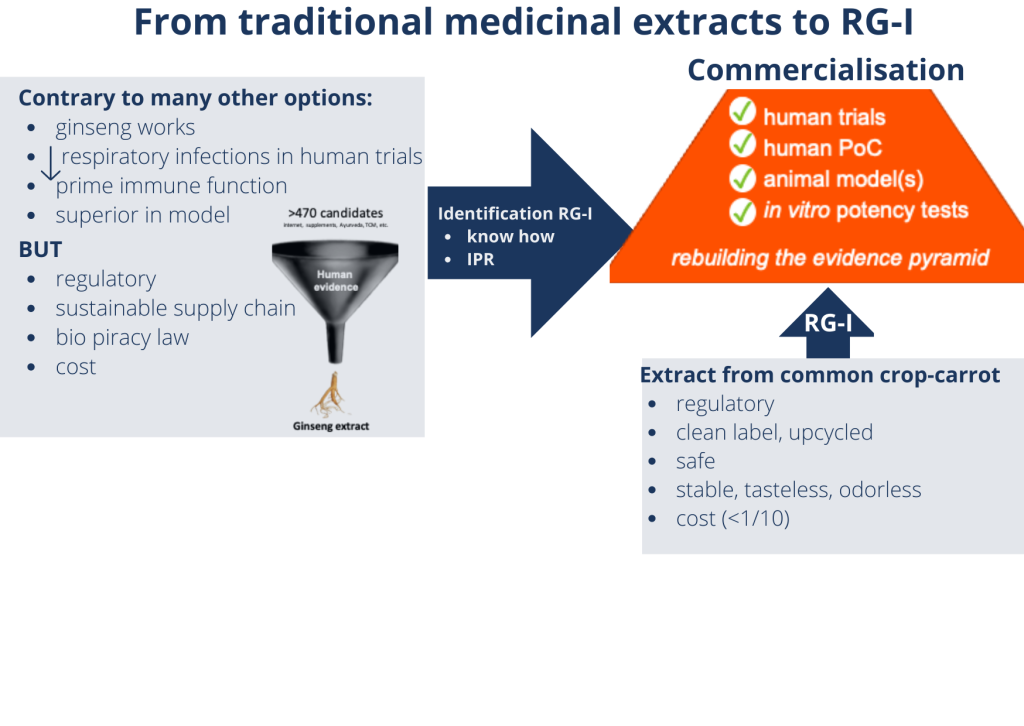
WH: What methods did you use to prove the effectiveness of the ingredient?
RA: Just as you would do to prove the effectiveness of a fire brigade, we set a ‘fire’. Together with NIZO and the Amsterdam University Medical Centre, we carried out a Rhinovirus challenge study in which 146 healthy volunteers consumed the ingredient (or a placebo) for eight weeks. Subsequently, they were administered a standardised amount of an attenuated common cold virus in their nasal cavity. We then monitored the development of (mild) cold symptoms and assessed various aspects of the immune response to the viral challenge.
The initial results of the trial, which have been published, showed a significant acceleration of protective innate antiviral responses, and reduced severity and duration of cold symptoms by 20 and 25%, respectively.
WH: Why did you choose a viral challenge test for the clinical trials?
RA: Viral challenge studies are the most compelling way to demonstrate improved immune health, although these studies are quite challenging to do. The model allowed us to demonstrate that our ingredient accelerates protective immune responsiveness and thus minimises the impact of the (model) infection on vitality (how well one feels and is able to perform daily activities). This keeps the ingredient’s immune health benefits within the regulatory boundaries for functional foods and supplements.
WH: How does your ingredient work?
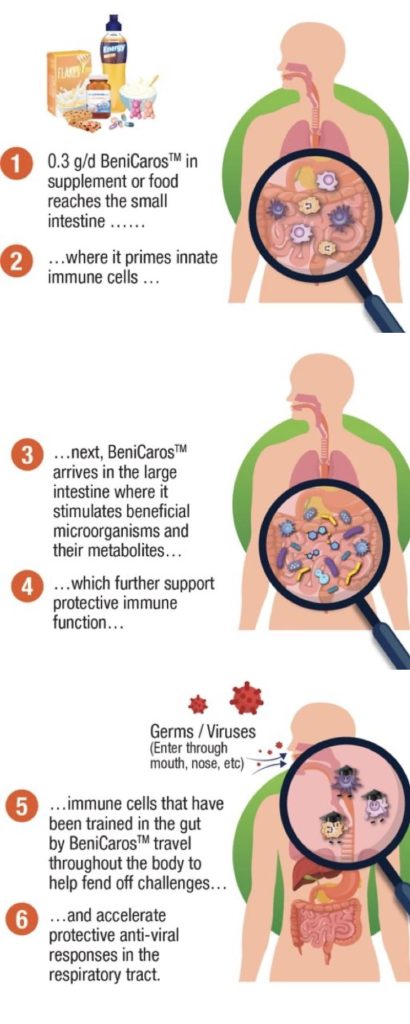
RA: The ingredient has a dual mode of action.
First is the direct effect on the immune system. Our ingredient’s complex chemical structure is stable in the stomach (small intestine) and can be sensed by cells of the innate immune system that actively monitor the content of the small intestine. These cells are thus primed, or ‘trained’. Some then migrate to the respiratory tract, passing on the lessons learned and improving local responsiveness.
The second mode of action is an indirect effect via gut microbiota modulation. The ingredient is fermented by the gut microbiota in the large intestine, leading to the production of metabolites such as short-chain fatty acids. These metabolites also support the immune system, which further improves responsiveness to infections.
WH: What sort of support did you need to develop your product and prove its immune health effect?
RA: As a small, knowledge-based company, we focus our efforts on driving our proprietary ingredients to commercialisation by setting up a supply chain, navigating the regulatory challenges, and demonstrating effectiveness. We do this with a small but very experienced team. At various stages, we turn to external experts to help us address challenges outside our core know-how. The support we needed for this project included not only the relevant capabilities and equipment but also a ‘can-do’ attitude that matches our own. It has been a long journey, and we are pleased that last month we launched BeniCaros® onto the US consumer market, together with Bayer.
The paper “The Dietary Intake of Carrot-Derived Rhamnogalacturonan-I Accelerates and Augments the Innate Immune and Anti-Viral Interferon Response to Rhinovirus Infection and Reduces Duration and Severity of Symptoms in Humans in a Randomised Trial” is available via the Nutrients open access journal.
In our next article, we will look at rapid screening for microbial functionality.
Related cases
Related blogs
© NIZO 2025 | Sitemap - Privacy Statement - Cookie Statement - Terms & Conditions
Website by: Online Marketing Agency