Table of Contents
In this series of articles, Will Chu, Science Editor for NutraIngredients, discusses some of the key issues and challenges facing the nutraceutical and food ingredient industry today.
Dietary fibre plays a key role in human health; fibre-rich foods and prebiotics can help promote gut health and benefit the gut microbiota, for instance. Adding fibre to foods and ingredients can thus help increase their attractiveness to consumers, and differentiate them in a competitive market. Petra Scholtens, Project team leader Nutrition & Health at NIZO, explains how in vitro models and human trials, supported by bioinformatics, can help manufacturers identify and demonstrate the health benefits of dietary fibres.
Will Chu: What is dietary fibre, and why is it important in the human diet?
Petra Scholtens: Dietary fibre is primarily the undigestible parts of plants. It can be found in vegetables, fruits, whole grains and legumes. Human milk also contains a type of dietary fibre – human milk oligosaccharides (HMO) – which underscores its importance for our health, even very early in life.
Certain fibres can support regular bowel movements; improve blood lipids, blood glucose regulation and mineral absorption; modulate the immune system; and control feelings of hunger and satiety. However, there is a great diversity of dietary fibres, and they do not all offer the same health benefits. Therefore, in a healthy diet, it is important to consume a variety of types of fibres.

WC: What is the relationship between dietary fibre and prebiotics?
PS: Many current prebiotics are indigestible dietary fibres. Prebiotics are defined as ‘a substrate that is selectively utilised by host microorganisms conferring a health benefit’. But while they cannot be digested by our bodies, they can be degraded by bacteria in our gut, which use digestive enzymes to ferment the fibres. Around 10,000 of these enzymes are found in the genome of gut bacteria, compared to only 8-17 in the human genome.
Sometimes, bacteria in the gut work in tandem: one type performs a first fermentation, which breaks down the fibre enough for other types of bacteria to perform a secondary fermentation. Together, these microbes in our gut may then be able to use the degraded fibre to produce beneficial compounds, such as short chain fatty acids. In addition, fibre can induce a shift in the gut microbiota composition, which also may have a direct benefit, such as by stimulating beneficial species at the expense of potentially pathogenic species. Adding specific types of dietary fibre is thus one possible way to provide a prebiotic effect in a food or ingredient.
WC: What does a manufacturer need to do to make a claim about a product or ingredient containing a fibre?
PS: It depends on the claim you wish to make. ‘High in fibre’, for example, is a nutrition claim related to fibre content, and therefore does not need to be backed up with additional supportive evidence. But if you want to make a specific health claim – such as lowering cholesterol or supporting the immune system – you need support from scientific studies. EU-approved fibre claims include arabinoxylan, which reduces postprandial blood glucose; and barley, oat grain and wheat bran fibres, which increase faecal bulk.
For some fibres, an effect on the gut microbiota has already been demonstrated in multiple studies: inulin, fructooligosaccharides (FOS) and galactooligosaccharides (GOS), for example, have been shown to have an impact on the composition of the intestinal microbiota, including stimulation of bifidobacteria.
But other, complex fibres, from lentils or grains, for example, might stimulate a larger variety of beneficial gut bacteria compared to the “traditional” options. This makes them very interesting possibilities for adding health benefits to a food or ingredient. In such cases, further investigation is necessary to prove a health impact.
WC: What types of tests or studies can be used to evaluate potential health benefits?
PS: The great diversity of both fibres and gut microbiota makes evaluating the health benefits quite complex, but there are several in vitro approaches that can be used. You can start with a simpler high-throughput model to quickly and cost effectively screen a large number of possible fibres and identify those with potential. Then you can move on to more complex, dynamic, multi-compartmental models, such as SHIME, TIM-2 and PolyFermS, to determine some potential health benefits for closer investigation. You can see which bacterial species are stimulated, what metabolites they produce, whether short chain fatty acid production is stimulated, etc.
In an in vitro setting, it is also possible to combine two differentmodels. You might, for instance, start with a high-throughput fermentation model, followed by a gut health in vitro model, such as a cell assay that focuses on barrier integrity using Caco-2 cells.
As an example of what type of information you can get from in vitro studies, a few years ago NIZO played a role in a study about the possible prebiotic effects of cRG-I, a carrot-derived pectic polysaccharide. Using an in vitro approach, the study showed that cRG-I selectively modulates the human gut microbiota while promoting gut barrier integrity, making it ‘a promising prebiotic candidate to proceed to clinical studies’.
However, in vitro models lack the real-time, human part of the equation: the immune factors, the reabsorption of metabolites and other nutrients, etc. That is where clinical trials come in.
WC: How do you test for health effects of dietary fibres in clinical trials?
PS: Demonstrating a true causal effect of dietary fibre on health requires a randomised clinical trial, for instance. But in a human setting, it is difficult to connect a specific fibre to a specific effect, because it depends on the microbiota present in the subjects, which is highly personal. You therefore need a relatively large sample size to cover this variation. You also should include a control group that receives a product without the fibre. And the diet of the participants must be controlled very closely to prevent bias from other dietary components
But a carefully designed clinical trial allows you to look at both the fibre’s mechanistic factors and physiological effects. The mechanistic factors may include the composition of the microbiota, and an assessment of the short fatty acids and certain immune parameters. The physiological effects can range from stool consistency and frequency to the immune or other health benefits being investigated.
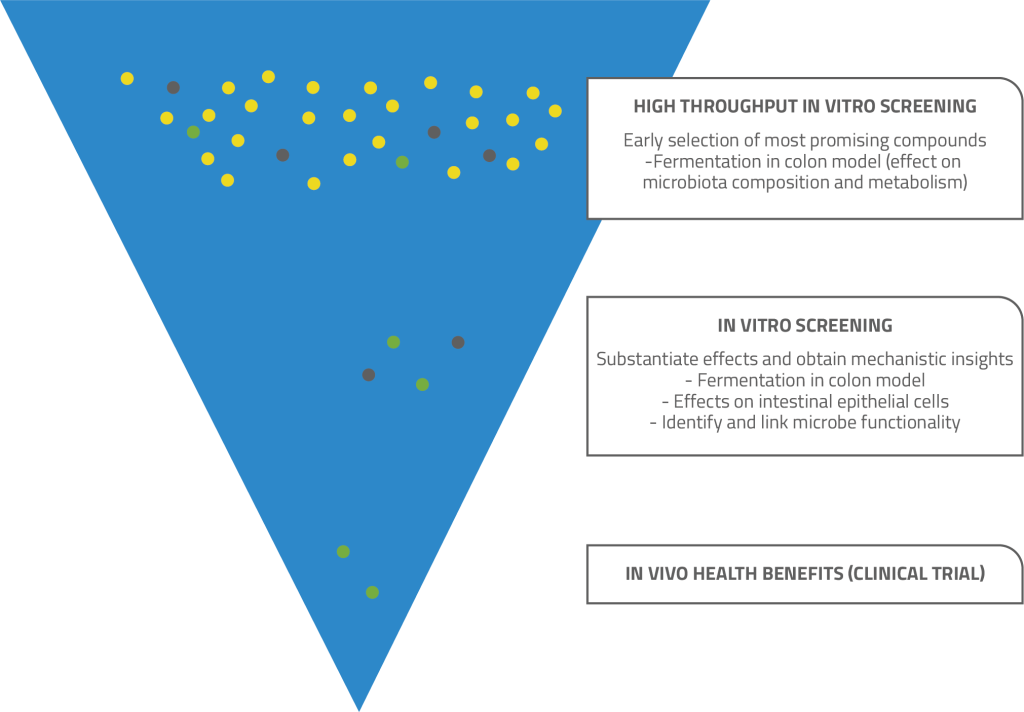
Schematic visualisation for screening and health benefit substantiation of dietary fibres with prebiotic potential
WC: What role can bioinformatics play in these trials?
PS: In both in vitro and in vivo studies, effects on the microbiota composition and its functions can be studied by metagenomic sequencing of the faecal DNA. For that, we need bioinformatics to analyse and visualize the effects. Combined with biological knowledge, it helps for the understanding of the changes in the microbiome: which microbes are inhibited, which are stimulated, what they may possibly do, for example. We can also link the effects to clinical outcomes. Finally, we can use bioinformatics more and more to demonstrate specific functionality, such as screening for microbial genes that encode the specialised enzymes needed for complex fibre degradation.
In our next article, we will look at ‘Single cell biomass – how might it contribute nutritive value and what is its health potential?’
Related cases
Related blogs
© NIZO 2025 | Sitemap - Privacy Statement - Cookie Statement - Terms & Conditions
Website by: Online Marketing Agency